so…what’s really in my acne cream/lotion?
by crumpetsandt
soooo i was going to write about favorite, highly recommended gel eyeliner today (put off until tomorrow) but i thought i’d do a little chemistry demystifying of acne cream and lotions. i think most people don’t really know what active ingredients are in their pimple stuff and it would benefit the average consumer to be aware of what s/he is really slathering onto her/himself. note that i will only be giving chemical analysis NOT medical analysis, so gather ’round kids and let’s begin!
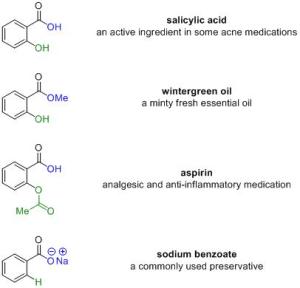
the two most commonly used active ingredients in over-the-counter (OTC) acne medication (meaning non-prescription) are salicylic acid and benzoyl peroxide. each of these act as acne medication in different ways, meaning each lessens acne lesions via different chemical mechanisms. let’s first look at the chemical structures (pictures, figure 1 and later 2) of each to see and understand more of how these chemicals work:
so in figure 1, i’ve shown salicylic acid with three other well known compounds wintergreen oil (mmm minty), aspirin, and sodium benzoate. it’s quite cool to take a look at how the ingredients look like structurally to see why they might behave similarly. as you can see salicylic acid looks very similar to wintergreen oil and they differ only by the blue parts of the molecule (OH vs OMe). you don’t need to know what these letter combinations mean but it makes sense that both of these molecules when rubbed on skin give a burning sensation (a little different between the ouch burning of salicylic acid versus the tingly burning of wintergreen oil). similarly if you take a look at salicylic acid and aspirin, you see that the blue parts are the same (both OH) but the bottom right parts in green are different. this similarity gives them properties/modes of actions that react with the human body as an anti-inflammatory. although the biological pathways of these two molecules as an anti-inflammatory is complex, one of the modes is via acting as a proton (via the carboxylic acid group) shuttle in the mitochondria (remember high school bio?). pretty nifty, no? lastly, sodium benzoate differs the most from salicylic acid. it is the sodium salt of the acid (see blue part) with no OH group (see green part). it is often used as a food preservative or cosmetics preservative. while we’re on the topic of sodium benzoate, i want to clarify the “scare” that sodium benzoate turns into benzene (a known carcinogen) when mixed with vitamin c (ascorbic acid). while the fda article is talking about the scare of sodium benzoate in soft drinks turning to benzene in the presence of vit. C, which in my book i have much beef with if you look at the data but more another day, i want to say that sodium benzoate in your cosmetics will not turn into benzene to kill you/cause you harm even if your moisturizer/makeup product contains vitamin c. i’ve seen on too many “health/natural living” beauty blogs give ABSOLUTELY wrong information about this (about how sodium benzoate will decompose to benzene in the presence of vitamin c) and this claim is chemically craaaaazy. if you can really prove that, let me know and we’ll write a jacs/nature/science paper together!
in figure 2, we see that benzoyl peroxide looks completely different than salicylic acid. it has in the middle two oxygen atoms that are connected by a line (a bond) and these types of molecules are known as peroxides. now certain peroxides can explode because that line in between the oxygens (the bond) can break apart too quickly/too much at once. with benzoyl peroxide and hydrogen peroxide they are relatively stable than other peroxides, especially because they are often formulated in some sort of liquid (as a solution or a lotion/cream/gel). these two peroxides are used often as disinfectants for the human body and react primarily through breaking of that bond to generate what are called free radicals. thus these radicals kill off bacteria by reacting with the bacteria’s survival machinery (cell wall, organelles, etc.). unfortunately, because the radicals generated aren’t specified to only attack the molecules in bacteria, it may also react with other things like cloth (causing bleaching) or yikes, the person’s cells leading in some cases to an adverse reaction (swelling, burning, allergy). although people can also be sensitive/allergic to salicylic acid, it makes sense that the reactive nature of benzoyl peroxide would cause more of a reaction than the acid acne ingredient.
most sensibly, start off using salicylic acid in your acne medication (careful not to use too much as it can cause a chemical burn) and if your doctor tells you to use benzoyl peroxide products, use carefully (don’t over medicate!) and with caution. benzoyl peroxide, while is okay to use in controlled amounts as directed by a physician, is not a compound/chemical to be trifled with and carelessly slathered on.
hope this helps clarify what’s in your jar of acne cream and may you have clear skin in the days to come!


OMG someone had to do the research. Thank you!! I BP does not work on me 😦
Jinelle
http://jinelleloves.wordpress.com/
no problem!
this is soo good. did you draw the figures in chem draw and color code them?
YES! i figured for the general audience (non-orgo ppl) it’s easier to say “look at the colored parts, they’re different!” instead of explaining all the technical stuff and confusing people. i think it’s pretty cool to write about something that i get to do everyday (chemistry) and make it relevant to what i love (beauty/fashion) =D